Design and synthesis of valence tautomeric compounds
 分类:research
分类:research
 作者:
作者:
 发布时间:2019-05-13
发布时间:2019-05-13
Valence tautomerism (VT) is rarely observed in transition-metal compounds. The occurrence of VT involves intramolecular ligand-metal electron transfer and simultaneous metal-ion spin crossover, typically when the system contains redox-active quinone-type ligand in the dianion form (Cat2−) or radical anion form (SQ●−). The quinone ligand orbitals generally lie close in energy to the metal antibonding orbitals and can donate electrons to or accept electrons from the metal ion (Figure 1). So, VT is in fact a kind of intramolecular redox reaction in nature. Because the temperature-dependent magnetic moments of VT compounds resemble those of SCO, VT can be viewed as a special case of SCO.
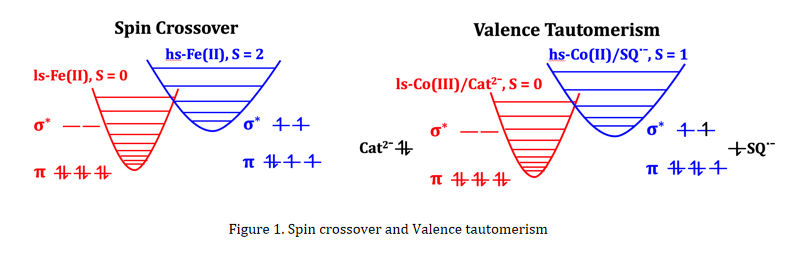
VT has been reported in compounds containing a variety of metal ions (such as Co, Mn, Cu, Fe, Rh, Ir, etc.), but more than 90 percent VT compounds are built from Co ion in the form of hs-[CoII(SQ)N4] or hs-[CoII(SQ)2N2] (high-spin phase). Taken hs-[CoII(SQ)N4] as an example, as temperature decreasing the Co(II) ion will donate an electron to the radical ligand SQ●−, which is then transformed into a dianion form Cat2−. Simultaneously, the resulted Co(III) ion is converted to the ls state. Such VT transition between paramagnetic hs-[CoII(SQ)N4] and diamagnetic ls-[CoIII(Cat)N4] is usually triggered by temperature variation, and the magnetic properties of the two tautomeric forms are quite different.
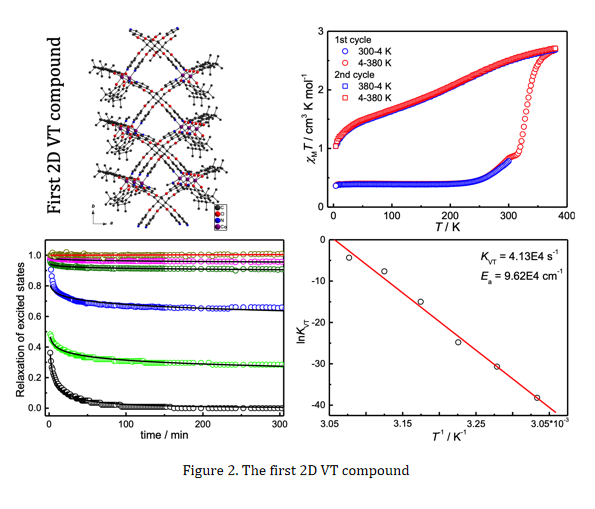
In our pursuit of VT compounds, we have mostly adopted 3,5-di-tert-butylcatechol (3,5-DBCat) as the redox-active ligand and synthesized VT compounds through in situ formation of its radical isomer (3,5-DBSQ). For example, the reaction of 3,5-DBCat, cobalt(II) acetate and tetrakis(4-pyridyloxymethylene)methane (tpom) in ethanol-water solution resulted in the formation of the first 2D VT compound {[CoIII(3,5-DBCat)(3,5-DBSQ)]2(tpom)}∙8H2O∙2C2H5OH. This compound is in the ls state, ls-CoIII-Cat2−-SQ●−, and transformed to its hs state, hs-CoII-(SQ●−)2, upon temperature increasing to 380 K (Figure 2). The photo-excited phase could be stable for hours to days.