Development of spin crossover-fluorescence materials
 分类:research
分类:research
 作者:
作者:
 发布时间:2019-05-13
发布时间:2019-05-13
The spin-state switching in spin-crossover (SCO) compounds often results in significant changes of structural and physical properties. These changes may simultaneously induce variations in other properties of different species in the same system and vice versa, that is, multifunctional materials with synergetic effects can be obtained.
In fact, multifunctional materials of synergetic SCO-other properties have long been sought, of which SCO-fluorescence is well studied because fluorescent emission and spin-state switching (ferrous ion) can intrinsically correlate with each other. As shown in Figure 1, fluorescence occurs when an orbital electron of a molecule, atom, or nanostructure, relaxes to its ground state by emitting a photon from an excited singlet state. The relaxation is a energy dissipating process, either by emitting a photon or by non-radiative (heat) or by conversion to a triplet state (phosphorescence) or by a secondary non-radiative relaxation step. If a second molecule or atom is present in the system, relaxation from the excited state can also occur through fluorescence quenching, which means that energy can be transferred from fluorophore to fluorescence quencher. Interestingly, the excited states of ls-Fe(II) ion have a singlet state 1T1, which can act as the fluorescence quencher given that it is close to the energy level of excited state of fluorophore. So, we may expect that the occurrence of SCO (or spin states) and fluorescent emission can manifest each other, and thus multifunctional materials with synergetic effects are obtained.
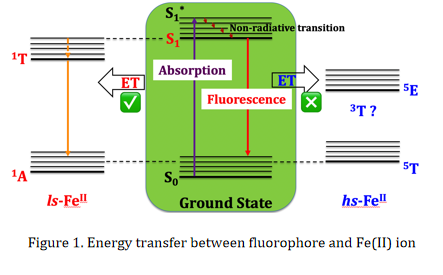
If energy transfer does occur between fluorophore and ls-Fe(II) ion, fluorescence is quenched at low temperature where Fe(II) ion is in the ls state and then achieves its maximum at a temperature when Fe(II) ion just switches to the hs state. Thus, this kind of materials can be used as 'molecular thermometer'.
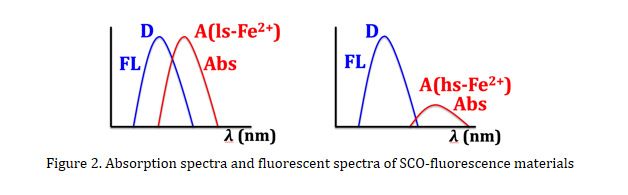
In order to verify the occurrence of energy transfer in SCO-fluorescence materials, variable-temperature absorption spectra and fluorescent spectra are usually measured. At low temperature (often at the ls state of ferrous ion), the fluorescent spectrum overlaps the absorption spectrum of Fe(II) ion that is commonly assigned to the LMCT and/or d-d transition, indicating the possibility of energy transfer. While at high temperature (usually at the hs state of ferrous ion) the overlap is negligible (Figure 2). The synergy between SCO and fluorescence can then be shown by the measurements of magnetic susceptibilities and fluorescence.
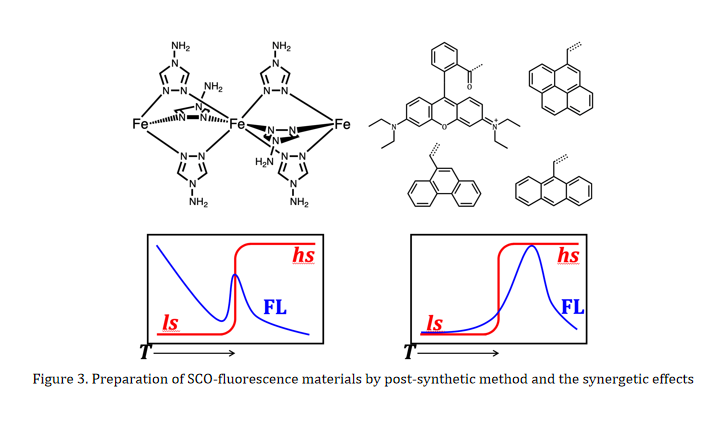
The preparation of SCO-fluorescence materials includes physical mixture and chemical synthesis. Our strategy is to obtain such materials by chemical synthesis, such as synthesizing SCO compounds with fluorescent ligands or post-modification of SCO compounds with fluorophores. For example, we recently prepared a series of SCO-fluorescence materials by post-modifying a well-known one-dimensional SCO compounds with various fluorophores through aldimine condensation reaction (Figure 3). Measurements on the variable-temperature magnetic susceptibilities and fluorescence showed that the spin-state switching and fluorescent emission may interact in two synergetic ways.